Frequently Asked Questions (FAQ)
Acronyms
APC - Aerobic Plate Count
CFU – Colony Forming Unit
cm2 – Centimeters Squared
Coli – Total Coliform (not E. coli)
FDA – United States Food and Drug Administration
HPC – Heterotrophic Plate Count
IMS – Interstate Milk Shippers
M&Y – Mold & Yeast
mL – Milliliter
NES – Number Exceeding Standard
PDA – Potato Dextrose Agar
PEW – Pasteurized Equivalent Water
PMO – Pasteurized Milk Ordinance
RBC – Residual Bacteria Count
RCC – Residual Coliform Count
RODAC – Replicate Organism Detection and Counting
SMA – Standard Methods Agar
Spp – Species
TC – Temperature Control
TBC – Total Bacteria Count
TNTC – Too Numerous To Count
TPC – Total Plate Count
VRBA – Violet Red Bile Agar
Collection & Submission Procedures
Test
Type
Disintegration
Pour Contact
Silicone
Rinse
Swab
Water
Sample Quantities
Sampling Protocols
Swab Culturette
Petri Dishes
Report Examples
FAQ
Q: Our test report shows results "<1" or "<4" , or some other “less than” ( < ) value, is that the same as "0" ?
A: The "less than" ( < ) notation is used to indicate that statistically, no colonies or evidence of growth were seen on the plate. However, there is variability in the microbiological testing process. This variability comes from several sources - sampling by the client, sub-sampling by PCI, test processing, plate counting, etc. For that reason, while no colonies were seen and counted, the Laboratory Technicians will represent the test result as "less than" a value.
Given the sample, the testing and the inherent variability, the Laboratory Technicians were not able to extract bacteria from the sample, grow and then find any colonies - but cannot statistically state that they were not there. We can only state that the results were less than an amount determined by the dilution used in the test, or by the test procedure itself.
Q: What are your accreditations?
A: We are a US FDA IMS (Interstate Milk Shipper) certified laboratory. We maintain this certification through New York State Department of Agriculture and Markets laboratory evaluation and proficiency programs. We also hold ISO 17025 accreditation through A2LA. Please see our Certifications & Links tab for a copy of our certificate and scope of accreditation.
Our Single Service Consultants also hold certifications from the US FDA and copies of their SSC Standardization certificates can also be found under Certifications & Links.
Q: How often should we submit product samples?
A: This varies based on the sample type and application, but in most cases, samples should be submitted at least 4 times in 6 months per machine/line. This breaks down to about once every 6 weeks.
Packaging bacterial standards and submission frequency can be found in Section C of APPENDIX J. STANDARDS FOR THE FABRICATION OF SINGLE-SERVICE CONTAINERS AND/OR CLOSURES FOR MILK AND/OR MILK PRODUCTS from the Grade “A” Pasteurized Milk Ordinance, U.S. Department of Health and Human Services, Public Health Service, and Food and Drug Administration, current revision. Please see our Certifications & Links page for a link to this reference.
Q: How do I collect product samples?
A: Refer to our sampling protocols listed under the “Collection & Submission Procedures” section. Any “irregular” unlisted items may require a phone inquiry for instructions.
Q: How much product sample is needed for testing?
A: Refer to our “Collection & Submission Procedures” section for various sample types. Contact us if you do not think your sample fits any listed category or are unsure.
Q: What is the correct way to send in product samples?
A: Refer to the “Collection & Submission Procedures” section of FAQs and the “Forms” page for the sample submission form. Contact us if you need assistance completing the form.
The submission form should contain identifying information for
the product sample(s) to be reported as well as report recipient contact information.
Q: How do we determine the testing methodology used?
A: In general, the testing methodology varies based on the type of sample. Samples are tested in accordance with applicable sections from Standard Methods for the Examination of Dairy Products, 17th ed., American Public Health Association, 2004. The actual section referenced can be found on the sample test report, where applicable.
For example, per the scope found in Standard Methods, the Rinse test is applicable to firm-walled, uncapped items, flexible-walled items, individual items of equipment, and CIP assemblies. This applies to containers that can hold liquid for us to flush a measured volume of liquid in the container and then plate the solution to enumerate the bacterial population. The Swab Contact Method is applicable to food contact surfaces that are not smooth or readily accessible. With this method, we apply a sterile, moistened swab over a measured packaging surface area. Place it in a known volume of liquid broth which is then plated for bacterial enumeration.
The Disintegration method is applicable to paper, paperboard, or mold pulp items in intermediate or complete stages of conversion. The Pour Contact test is applicable to flat, nonporous food contact surfaces.
Q: What are acceptable water results?
A: Acceptable sanitary water supplies will be absent of coliforms (ex. <1 CFU/mL). Currently, there are no regulatory standards for bacteria found in water. Therefore, bacterial water standards must be derived from an internal risk analysis or as determined by your customer(s). We recommend resampling if a result of <1 CFU/mL is required internally for bacteria in addition to the absence of coliforms.
Some factors for consideration when trouble-shooting results exceeding internal standards may include water sample type (potable or from some other source) and reviewing the sampling method. Was the sample port, faucet, or other means of collection sanitized? Refer to our sampling protocols listed under our “LINKS” tab. Any “irregular” sample sources or sampling methods may require a phone inquiry for instructions.
Q: Is little to no bacteria found in/on a product sample an indicator of lack of mold and yeast as well?
A: Little to no bacteria does not directly indicate that you wouldn’t have mold or yeast present. Although it may indicate that the sample or environment is relatively sanitary, it cannot directly indicate that there are no mold or no yeast spores present to potentially contaminate the (packaging) sample even when there are no bacteria found. We offer mold/yeast testing in addition to our standard total bacteria and total coliform testing.
Q: Did our product samples with counts pass?
A: Most acceptable regulatory standards can be found in the “Standards” section on your test report and vary based on the test type used. However, environmental test results for petri dishes and swab culturettes, mold & yeast testing, along with bacterial results for water will be determined internally by your organization or as required by your customer specifications. Your organization or customer(s) may also have additional or more stringent microbiological testing standards to meet internal Quality & Food Safety Programs specifications.
Packaging bacterial standards can be found in Section C of APPENDIX J. STANDARDS FOR THE FABRICATION OF SINGLE-SERVICE CONTAINERS AND/OR CLOSURES FOR MILK AND/OR MILK PRODUCTS from the Grade “A” Pasteurized Milk Ordinance, U.S. Department of Health and Human Services, Public Health Service, and Food and Drug Administration, current revision. Please see our Certifications & Links page for a link to this reference.
Q: What are acceptable results for a Disintegration Test?
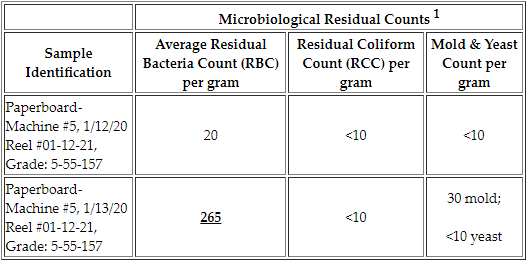
A: The residual bacteria count for the Disintegration Test is not to exceed 250 colonies/gram when used in a dairy application and should be absent of coliform.
Refer to our sample reports in the Collection & Submission Procedures section above for additional details or contact us with any other inquiries.
Example:
The first paperboard sample has all acceptable results. However, the second sample has a Residual Bacteria Count (RBC) of 265 bacteria colony-forming units (CFU). This count exceeds the maximum regulatory standard of 250 bacteria CFUs for products that will be used in a dairy application in adherence with the Grade “A” Pasteurized Milk Ordinance. See our Certifications & Links page for a link to this reference. Mold and yeast standards are determined internally by your organization or by your customers’ specifications.
Q: What are acceptable results for a Pour Contact Test?
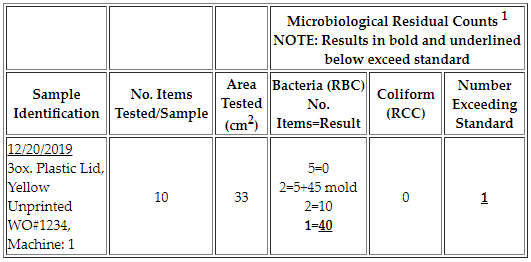
A: The residual bacteria count for a Pour Contact Test is not to exceed one (1) colony per square centimeter of surface area tested (ex. <1) and should be absent of coliform.
The bacteriological count cannot exceed the surface area tested (ex. 33 cm2). Refer to our sample reports in the Collection & Submission Procedures section above for additional details or contact us with any other inquiries.
Example:
1 out of 10 sample items tested for a lid with an area of 33 cm2 has a residual bacteria count of 40 bacteria colony-forming units (CFU). All other counts are less than 33 CFU, except an item with a mold count of 45 CFU. However, mold and yeast standards are determined internally by your organization or by your customers’ specifications. As a result, only one sample exceeds the regulatory standard.
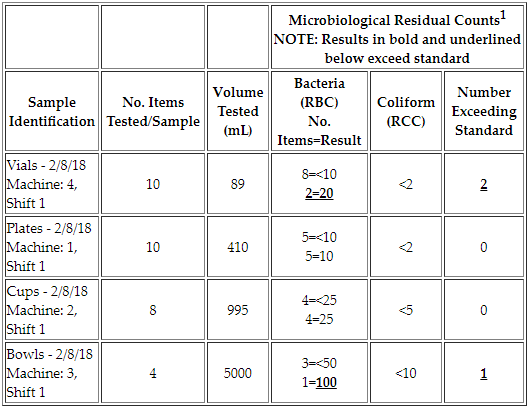
Q: What are acceptable results for a Rinse Test?
A: The residual bacteria count for a Rinse Test is not to exceed fifty (50) bacteria per item tested if the sample volume is ≥100 mL or ≤10 bacteria per item tested if volume is <100 mL and should be absent of coliform.
Refer to our sample reports in the Collection & Submission Procedures section above for additional details or contact us with any other inquiries.
Example:
Sample 1 (Vials) has a volume of 89 mL. As a result, 2 items exceed the standard of 10 residual bacteria for this smaller size container.
Samples 2 (Plates) & 3 (Cups) are all acceptable given the results in respect to their container volumes. However, sample 4 (Serving Bowls) has one sample that exceeds the standard for residual bacteria since one sample has a count of 100 CFUs and the maximum count is 50 for that container volume.
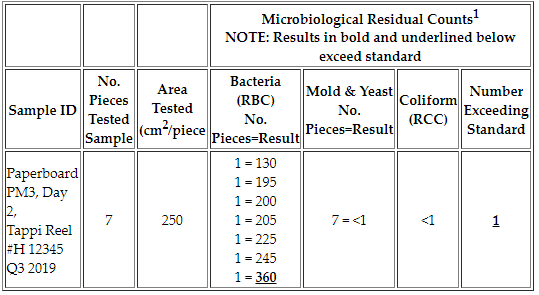
Q: What are acceptable results for a Swab Test?
A: The residual bacteria count for a Swab Test is not to exceed one (1) colony per square centimeter of surface area tested (ex. <1) and should be absent of coliform.
The Residual Bacteria Count (RBC) cannot exceed 250 colonies when an area of 250 cm2 from a minimum of 7 pieces (ex. sheets of paper) are tested since 250 cm2 is swabbed per piece/sheet, or the results should not exceed any other area when a smaller area is tested (ex. 50 cm2). Refer to our sample reports in the Collection & Submission Procedures section above for additional details or contact us with any other inquiries.
Example: 250 cm2 swabbed on each of the 7 pieces tested; 1 piece tested exceeds the bacterial standard with a count of 360 bacteria colony-forming units (CFU). The limit for this sample is 250 residual bacteria per piece since 250 cm2 were swabbed per piece.
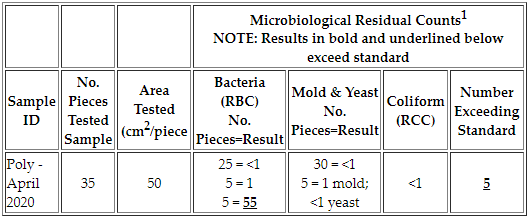
Example: 50 cm2 swabbed on each of the 35 pieces tested; 5 pieces tested exceed the bacterial standard with a count of 55 bacteria colony-forming units (CFU). The limit for this sample is 50 residual bacteria per piece since 50 cm2 were swabbed per piece.
Q: What are acceptable results for mold & yeast testing?
A: Currently there are NO regulatory standards for mold and yeast in food packaging. These standards will be determined internally by your organization OR as required by your customer specifications. Your organization OR customer(s) may also have additional or more stringent microbiological testing standards to meet internal Quality & Food Safety Programs specifications.
Q: Can you tell me what type of bacteria you observed?
A: We do not perform speciation testing, only total residual bacteria. However, we do work with another commercial laboratory that can perform this testing if requested.
Q: How often should we perform environmental ambient air testing and compressed air testing?
A: We recommend quarterly air testing. Standard Methods Agar (SMA) is used for bacteria testing and Potato Dextrose Agar (PDA) is used for mold/yeast testing. Each sampling location would be tested for bacteria using one SMA petri dish and one PDA petri dish for mold/yeast.
Q: What are the units of measurement for air plates?
A: The units of your environmental air plates are “Colony Forming Units/Plate” (CFU) since they have been exposed to the air and not inoculated with any known volume of air, sample, or diluent. The petri dishes used for air testing are 57 cm2.
Q: What are acceptable air testing results?
A: FDA has not identified a specification for the levels of bacteria, mold or yeast in air. While there are guidelines for what a facility might want to achieve with respect to total counts, these are only guidelines. Your standards will be determined internally or by customer requirements and interpretations will be based on historical data.
An approach that has been accepted is for a facility to state in its quality/food safety documentation what its expectations for environmental air and/or compressed air are, and the frequency of testing. Suggested verbiage could look like:
“The following specification is effective immediately for the ambient/environmental air used in areas of the XXX production areas. The requirements for compressed air (or environmental air) are:
- Contains minimal microbiological content;
- For compressed air, humidity and water content equivalent to supplier specifications;
- Filtration compliant with supplier specifications.
Monitoring of the microbiological content of environmental and/or compressed air will be once/quarter. The data will be trended to identify any significant deviations or issues.”
This is generally documented as a note to file or within the utilities section of your food safety manual. This is very broad verbiage and we do recommend that breadth of statement - keeping in mind that there is NO FDA-identified specification in this area.
We also recommend thoroughly cleaning the sampling area and re-sampling to verify the effectiveness of your cleaning methods. A preventive control may include more frequent cleaning and increased preventive maintenance of HVAC systems and/or air filtration. Please contact us for more specific inquiries.
Q: What are acceptable environmental swab test results?
A: FDA has not identified a specification for the levels of bacteria, mold or yeast in the environment. Your standards will be determined internally or by customer requirements based on a risk analysis (ex. contamination risk associated with proximity to product contact surfaces) and interpretations will be based on historical data.
An approach that has been accepted is for a facility to state in its quality/food safety documentation what its expectations for environmental swabs are and the frequency of testing. Suggested verbiage could look like:
“The following specification is effective immediately for swab test results in areas of the XXX production areas. The requirements for are:
Ø Contains minimal microbiological content; (set limits for bacteria, coliform, and/or mold/yeast counts, absence of potentially pathogenic species)
Environmental swab monitoring of the microbiological content will be once/quarter. The data will be trended to identify any significant deviations or issues.”
This is generally documented as a note to file or within the utilities section of your food safety manual. This is very broad verbiage and we do recommend that breadth of statement - keeping in mind that there is NO FDA-identified specification in this area.
We also recommend thoroughly cleaning the sampling area and re-sampling to verify the effectiveness of your cleaning methods. Preventive controls may include more frequent cleaning and increased preventive maintenance, in addition to other food packaging safety programs in place. Please contact us for more specific inquiries.
Q: What does “estimated due to spreader growth” mean on test results?
A: Spreader growth is a description of a naturally occurring colony which grows across the plate including over other colonies if present. (Not the same as confluent growth, but similar in that it must be estimated due to the way the colonies are growing and may inhibit other colonies that could potentially increase the count observed on each agar dish).
A Spreader may be only one colony, normally present in the sample, but has happened to grow rapidly across the agar plate. These instances are not necessarily indicative of a high count, but simply of a special type of colony that grows on the agar plate. Colonies covering >25% of the plates need to be retested as the as the spreading organism can inhibit or grow over other micro-organisms that may be present.
Q: What does “estimated due to confluent growth” mean on test results?
A: Confluent growth means a bacterial growth that is not well spatially separated from other growth with no discrete colonies when performing microbiological analysis. As a result, it is difficult to distinguish separate Colony Forming Units (CFUs) from each other to provide an accurate Bacteria Count as the bacterial growth covers a significant portion of the test surface and appear to all originate from the same colony and may inhibit additional colonies of bacterial growth. In some test methods, dilutions can be made to help spread the bacterial colonies out for better quantification, but some of our reference test methods have specific volumes of dilution buffer or nutrient broth for our analyses that we are not able to modify.
Packaging Consultants International
1101 State Fair Boulevard
Syracuse, NY 13209
Get Directions »
Tel: (315) 468-6234
Fax: (315) 468-6235
Email: customerservice@pcilabs.com
N/A
N/A
N/A
N/A
Contact Us